 CCS
CCS CCS
CCSREACH Regulation (EC) 1907/2006 is a regulation of the European Union on chemicals and their safe use. REACH stands for Registration, Evaluation, Authorization and Restriction of Chemicals. It has been put into force since June 1, 2007.
The body responsible for managing REACH is European Chemicals Agency (ECHA). According to REACH, all chemicals manufactured in and imported into the EU are subject to the requirements under REACH regulation including registration, evaluation, authorization and restriction if certain criteria are met. A comprehensive regulatory framework is established within REACH to implement the information communication, tracking and supervision among the chemical manufacturers, importers and downstream users in the supply chain, for the purpose of reducing the use of hazardous chemicals and protecting human health and the environment.
Testing Scope
Substances of Very High Concern (SVHC) in REACH, cut off 17th Jan. 2023, update to 233 SVHC.
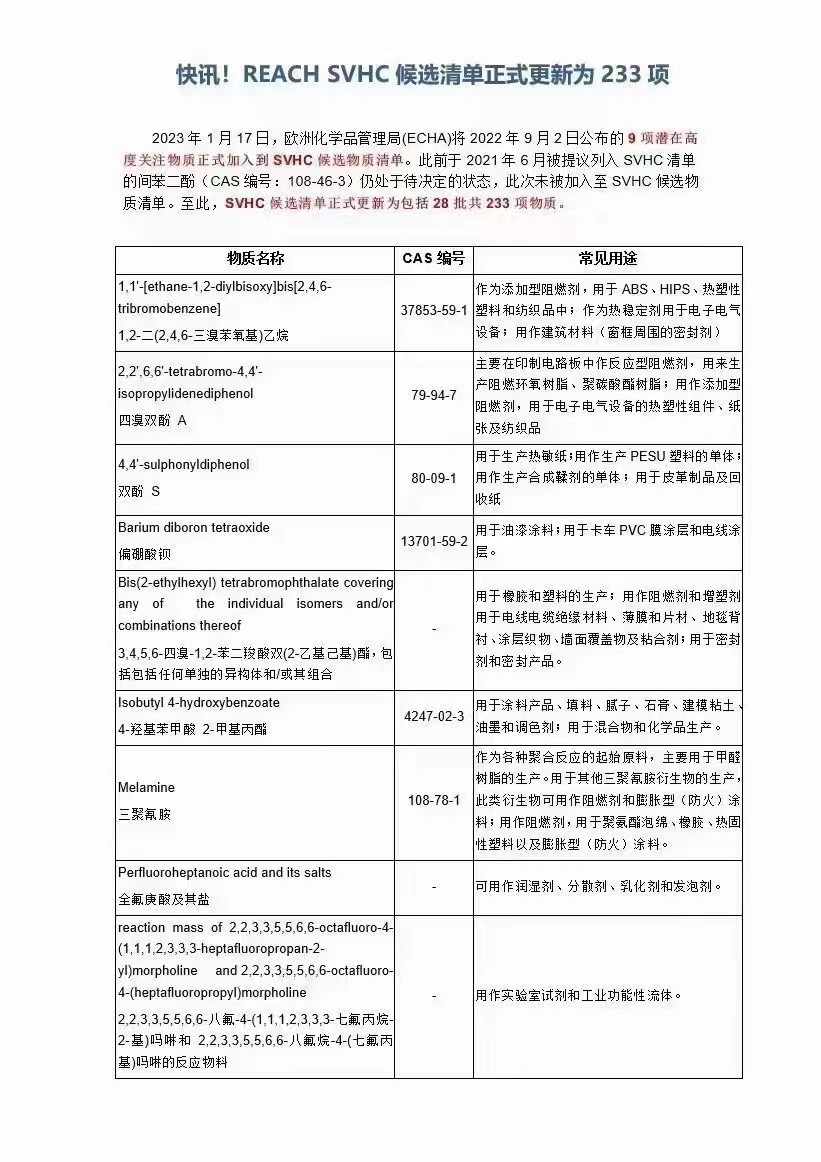
Any supplier of an article containing an SVHC in a concentration above 0.1% w/w has the duty to provide their customers with sufficient information of the article within 45 days upon request. The first list of SVHC candidate list for authorisation was released in 2008 and has been expanded several times to include new SVHC. In the future, this list will be further expanded.
REACH Compliance
1. Registration
The registration shall be submitted to the European Chemicals Agency (ECHA) by a natural or legal person in the EU or the "only representative" in the EU appointed by a ¡®non-EU manufacturer¡¯. Manufacturers and importers of chemicals shall submit a registration to ECHA with the relevant information of the chemicals involved and the safety measures required in handling the chemicals, if one of the following conditions are met:
¡ô Substances in quantities greater than 1 ton/year;
¡ô Article with substances (intended to be released) in quantities greater than 1 ton/year.
2. Evaluation
ECHA shall evaluate the registration dossiers and the substances
3. Authorization
Authorization is required for the substances classified as carcinogenic, mutagenic or toxic to reproduction (CMR) of categories 1 or 2; persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB).
4. Restriction
Certain hazardous chemical substances are restricted in the EU market through regulation under the conditions specified in the regulation. Those substances shall be integrated in the Annex XVII to REACH (the restricted substances in Annex XVII are continuously amended and updated).